-
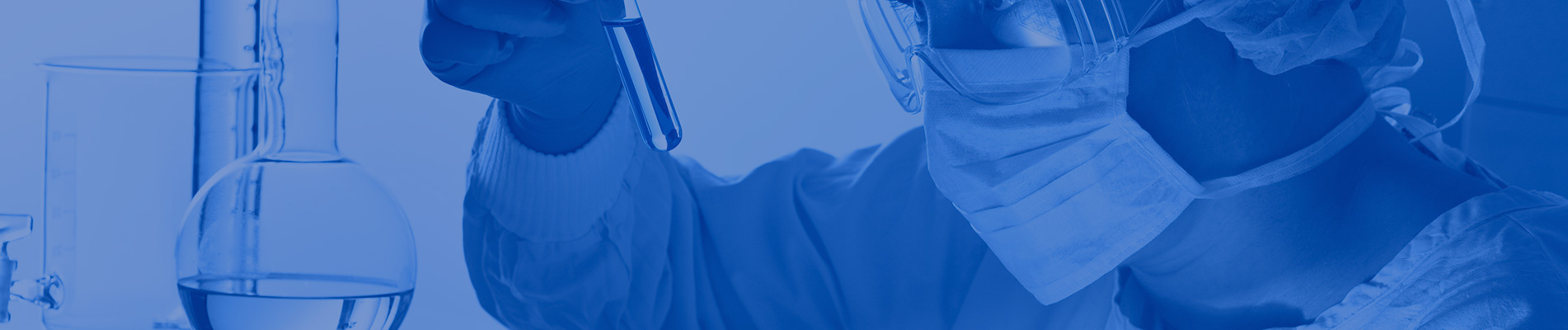
SARS-CoV-2 Antigen Rapid Test Device (Saliva)
INTENDED USEThe SARS-CoV-2 Antigen Rapid Test device is a rapid visual immunoassay for the qualitative, presumptive detection of SARS-CoV-2 antigens form Nasal swabs specimens. The test is intended for use as an aid in the rapid differential diagnosis of acute SARS-CoV-2 virus infection.PRINCIPLEThe detection of SARS-COV-2 adopts the principle of double antibody sandwich method and colloidal gold -
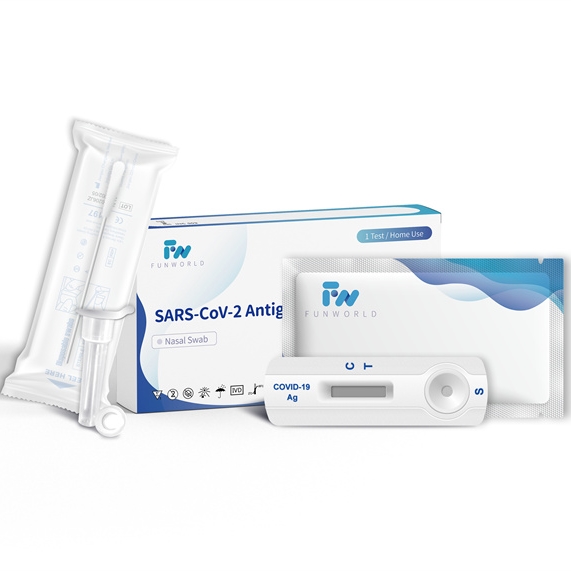
SARS-CoV-2 Antigen Rapid Test Device(Self-Testing)
PRODUCT SPECIFICATIONBrandFunworldCertificateCE / TGA / ISO13485/UK RegistrationSpecimenNasal swabPack1 test5 tests20 testsReading Time10 MinutesContentsCassette, Sterile Nasal Swab, Buffer ,Package InsertStorage2-30℃Shelf Life2 Years1.Specimen CollectionIt is important to obtain as much secretion as possible. Therefore, to collect a nasal swab sample, insert the sterile swab into the nostril that -

Multi drug Test Panel with CE ISO
One-step Drug Of Abuse Test KitsK2K2 One Step Synthetic Cannabis Test Device (Urine)50ng/ml, 30ng/mlAMPAMP One Step Amphetamine Test Device (Urine)1000ng/mlBARBAR One Step Barbiturates Test Device (Urine)300ng/mlBZOBZO One Step Benzodiazepines Test Device (Urine)300ng/mlBUPBUP One Step Buprenorphine Test Device (Urine)10ng/mlCOCCOC One Step Cocaine Test Device (Urine)300ng/mlCOTCOT One Step Cotini -

fFN Rapid Test Strip Manufacturer with Best Price
PROCEDUREfFN Rapid Test DeviceBring tests, specimens, buffer and/or controls to room temperature (15-30°C) before use.1) Remove the test from its sealed pouch, and place it on a clean, level surface. Label the testwith patient or control identification. To obtain a best result, the assay should beperformed within one hour.2) Add the extracted buffer into the sample well. As the test begins to work -

Funworld fFN Rapid Test Device for Wholesale
PROCEDUREfFN Rapid Test DeviceBring tests, specimens, buffer and/or controls to room temperature (15-30°C) before use.1) Remove the test from its sealed pouch, and place it on a clean, level surface. Label the testwith patient or control identification. To obtain a best result, the assay should beperformed within one hour.2) Add the extracted buffer into the sample well. As the test begins to work -

Human Fecal Occult Blood (FOB) Rapid Test Device
NTRODUCTIONColorectal cancer is one of the most commonly diagnosed cancers and a leading cause of cancer death in the United States. Screening for colorectal cancer probably increases the cancer detection at an early stage, therefore reduces the mortality.Earlier commercially available FOB tests utilized the guaiac test, which requires special dietary restriction to minimize false positive and fal -

Human Fecal Occult Blood (FOB) Rapid Test Strip
KIT COMPONENTSIndividually packed test stripsEach strip contains colored conjugates and reactive reagents pre-spreaded at thecorresponding regions.Specimens dilution tube withbuffer0.1 M Phosphate buffered saline (PBS) andpreservativePackage insertFor operation instruction.MATERIALS REQUIRED BUT NOT PROVIDEDSpecimen collection containerFor specimens collection use.TimerFor timing use.CentrifugeFor -

ALB Micro-Albumin Rapid Test Device/Strip (Urine)
Product ParametersProduct NameALB Micro-Albumin Rapid Test Device ALB Micro-Albumin Rapid Test Strip Used forFor professional in vitro diagnostic use only.SpecimenUrinePacking25 tests/box , 1 test/polybagMOQ1000 testsDiverse Coopetation ModesOEM/ODMPERFORMANCE CHARACTERISTICSThe accuracy of the Microalbumin test was evaluated in comparison to a commercially available immunoassay at a cut-off of 2 -

LH One Step Ovulation Test Strip/Device (Urine)
SUMMARY Ovulation is the release of an egg from the ovary. The egg then passes into the fallopian tube where it is ready to be fertilized. In order for pregnancy to occur, the egg must be fertilized by sperm within 24 hours after its release. Immediately prior to ovulation, the body produces a large amount of luteinizing hormone (LH) which triggers the release of a ripened egg from the ovary. This -

Adenovirus Rapid Test Device
PrincipleThe Adenovirus Rapid Test Device (Feces) has been designed to detect adenovirus through visual interpretation of color development in the internal strip. The membrane was immobilized with anti-adenovirus on the test region. During the test, the specimen is allowed to react with colored anti-adnovirus antibodies colloidal gold conjugates, which were precoated on the sample pad of the test. -

iGFBP-1 Rapid Test Device / Strip
PRINCIPLEThe iGFBP‐1 (vaginal secretion) has been designed to detect iGFBP‐1 thrisough visualinterpretation of color development in the internal strip. The membrane was immobilized withanti‐iGFBP‐1 antibodies on the test region. During the test, the specimen is allowed to react withcolored anti‐iGFBP‐1 antibodies colloidal gold conjugates, which were precoated on the samplepad of the test. The mix -

The hCG One Step Pregnancy Combo Test Device (Urine/Serum)
PRECAUTIONSPlease read all the information in this package insert before performing the test.Do not use after the expiration date printed on the foil pouch.Store in a dry place at 2-30°C or 36-86°F. Do not freeze.Do not use if pouch is torn or damaged.Keep out of the reach of children.For in vitro diagnostic use. Not to be taken internally.Do not open the test foil pouch until you are ready to sta