The earliest application of colloidal gold in medicine is to label antigens for electron microscope observation, mainly because it can combine with antigens, antibodies, sugars, and nucleic acids.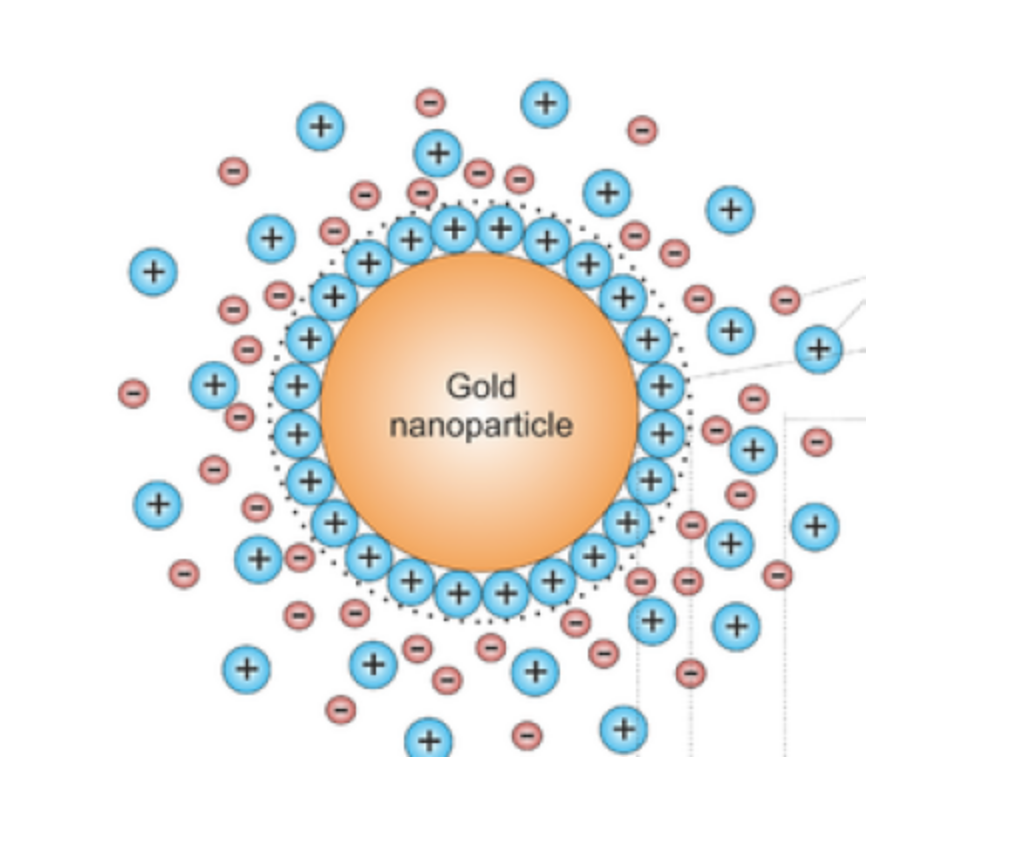
There are also many applications in biomedicine, such as drug delivery, gene therapy, etc. But more as a biosensor, such as SPR surface plasmon resonance gold film. Raman spectroscopic enhancers, etc. There are many uses of colloidal gold in medicine, which will not be repeated here.
We mainly discuss the application of colloidal gold in immunochromatographic detection. The principle of colloidal gold labeling of antibodies or antigens is mainly the electrostatic adsorption of gold particles and protein binding.
Therefore, to achieve good binding effect, the charge of the protein is an important factor. Therefore, when labeling, different pH values should be selected according to the isoelectric point of different proteins for labeling.
In the process of preparing colloidal gold, the key point is the stability of colloidal gold, which means that the prepared gold does not aggregate for a long time! In the preparation process, the details are often the key to success or failure.
Past experience shows that the vessel for burning gold is very important,
One is the material of the container, glassware is the first choice.
The second is the cleanliness of the container.
At present, few laboratories use the classic method of concentrated sulfuric acid and potassium dichromate to clean glass containers. Instead, they use a lot of detergent. What is detergent? It is the surfactant, and these residual substances are the enemy of colloidal gold.
In addition, the quality of the water is also an important factor. The measure of deionized water is conductivity. Therefore, in the system assessment, the conductivity is always used to make trouble, and the consistency of water quality is a hot topic.
Marking method: After the gold is prepared, it should not be marked in a hurry.
Three more things to do:
1. What protein to label, check the isoelectric point PI of this protein, and select a pH value higher than the isoelectric point for labeling.
2. Adjust the pH of colloidal gold.
High-salt disruption experiments to determine the saturating amount of labeled protein.
3. The pH value of colloidal gold is not adjusted by the traditional acid-base titration method, because strong acid and strong alkali tend to destroy the colloidal gold particles, and most of them are adjusted with 0.2M potassium carbonate.
The principle of the destruction experiment is based on the fact that high concentration of NaCl will destroy the bare gold particles, and the colloidal gold will turn purple.
If the surface of the gold particles is coated with enough protein, NaCl cannot destroy the gold particles, and the color is still red. Based on the amount of protein coating, an optimal label concentration can be determined.
Labeled gold must have a blocking step, and there are many blocked substances, including proteins, small molecular substances, polymers, surfactants and so on.
The purpose of blocking is to close the unbonded sites on the surface of gold particles, and to maintain the stability of colloidal gold on the other hand. The choice of sealing agent is also very knowledgeable
Post time:Jul-01-2022
Post time: 2023-11-20 16:50:58